First I am going to discuss common potent ICU drugs and break them down to their intracellular activities.

SODIUM NITROPRUSSIDE (SNP, NIPRIDE)
antihypertensive, vasodilator
USES: Sodium Nitroprusside also known as Nipride is a powerful parenterally administered vasodilator that is used in treating hypertensive emergencies as well as severe heart failure.
DOSAGE: 0.5-10mcg/kg/min
ACTION: Nitroprusside dilates both arterial and venous vessels, resulting in reduced peripheral vascular resistance and venous return. When infused intravenously Nipride interacts with oxyhemoglobin, dissociating immediately and forming methemoglobin and releasing cyanide and nitric oxide. Once released, nitric oxide activates the enzyme guanylate cyclase present in vascular smooth muscles, resulting in increased intracellular concentrations of cGMP. cGMP inhibits calcium entry into vascular smooth muscle cells (thus prohibiting muscular contraction) and may increase calcium uptake by the smooth endoplasmic reticulum to produce vasodilation.
CONTRAINDICATION: decreased cerebral perfusion, hypersensitivity
USE CAUTIOUSLY IN... renal disease (increased risk of thiocyanate accumulation), hepatic disease (increased risk of cyanide accumulation)
METABOLISM: Nitroprusside is a complex of iron, cyanide groups, and a nitroso moiety. It is rapidly metabolized by uptake into RBC's with liberation of cyanide. Cyanide is then in turn metabolized by the mitochondrial enzyme rhodanase, in the presence of a sulfur donor, to the less toxic thiocyanate. Thiocyanate is distributed in extracellular fluid and slowly eliminated by the kidney.

TOXICITY: the most serious toxicity is related to accumulation of cyanide. The healthy adult can eliminate cyanide at a rate equivalent to cyanide production up to approximately 2 mcg/kg/min. Toxicity may occur when infusion rate is >2 mcg/kg/min or when sulfur donors and methemoglobin are exhausted, allowing cyanide radicals to accumulate. Metabolic acidosis, arrhythmias, excessive hypotension, and death have resulted.
Administration of sodium thiosulfate as a sulfur donor facilitates metabolism of cyanide. Hydroxocobalamin combines with cyanide to form the nontoxic cyanocobalamin. Both have been advocated for prophylaxis or treatment of cyanide poisoning.
Thiocyanate may also accumulate especially in patients with renal insufficiency causing thiocyanate toxicity. This is manifested through weakness, disorientation, psychosis, muscle spasms, and convulsions.
NOREPINEPHRINE (LEVOPHED)
vasopressor
USES: Blood pressure control in acute hypotensive crisis. Norepinephrine is a sympathomimetic with potent alpha-1 and beta-1 stimulation. Unlike epinephrine, NE has very little beta-2 effect. When administered exogenously, NE stimulates alpha-1 receptors causing intense arterial and venous vasoconstriction, increased SVR, increased afterload, increased SBP, DBP, and MAP. Beta-1 stimulation results in positive (+) chronotropic (affects HR) and inotropic (affecting contractibility) effects causing increased HR, SBP, and subsequent myocardial oxygen consumption (although the + inotropic effects are limited due to the intense vasoconstriction).
Norepinephrine binding with alpha-1 and beta-1 receptors:

Alpha-1 receptor
1. NE binds with alpha-receptor
2. Gq protein is activated
3. GTP replaced GDP on alpha-subunit
4. Phospholipase C is activated
5. IP3 (inositol tri-phosphate) and DAG (diacylglycerol) are activated
6. DAG activates protein kinases
IP3 releases stored calcium leading to an increased concentration of free calcium
7. Free calcium binds with calmodulin
8. This complex activates myosin light chain kinase
9. The myosin light chain kinase phospholates the myosin chains on the myosin head in the presence of
ATP
10. This leads to a cross-bridge formation between the myosin heads and actin filaments causing smooth muscle contraction
*Increased SVR, MAP, CVP, PVR

Beta-1 Receptor
1. Norepi binds with beta-1 receptor
2. Gs protein is activated
3. Gs protein activates adenylyl cyclase to form cAMP from ATP
4. cAMP activates protein kinase-A
5. Protein kinase-A phospholates calcium channels increasing calcium entry into the cells
6. Increasing calcium during action potentials leads to increased calcium release by the sarcoplasmic reticulum in the heart (increasing contractibility, aka inotropy). Gs protein also increases HR by opening ion-channels responsible for pacemaker currents in SA node.
7. Protein Kinase-A phospholates sites on the sarcoplasmic reticulum which increases the release of calcium.
8. Calcium binds with triponin-C which enhances inotropy
9. Protein kinase-A phospholates myosin light chains. Myosin binds with actin. Myosin head flips forward causing contraction.
* Increased HR and contractility
DOSAGE: 2-20 mcg/kg
METABOLISM: Norepinephrine is metabolized by monoamine oxidase and catechol-O-transferase into vanillylmandellic acid and is mostly excreted in urine.
CONTRAINDICATIONS: HTN, PVD, use of MAOI's and tricyclic antidepressants, and lactic acidosis.
ADVERSE EFFECTS: digital gangrene, renal/hepatic/splanchnic/mesenteric necrosis, and hyperglycemia.
EPINEPHRINE
vasopressor, antiasthmatic, bronchodilator, adrenergic
SYNTHESIS and RELEASE: Epinephrine is synthesized from norepinephrine within the adrenal medulla. Preganglionic fibers sympathetic adrenergic nerves synapse within the adrenals. Activation of these fibers releases acetylcholine, which binds to postjunctional nicotinic receptors in the tissue. This leads to stimulation of NE synthesis within adenomedullary cells, but unlike sympathetic neurons, there is an additional enzyme (phenylethanolamine-N-methyltransferase) that adds a methyl group to the NE molecule to form epinephrine. The epinephrine is released into the blood perfusing the glands and carried throughout the body.
Epinephrine is a potent vasoconstrictor and cardiac stimulant. It has more beta-1 stimulation than norepinephrine.
-When administered in small dosages (1-2 mcg/min) = beta-2 stimulation
-When administered in moderate dosages (4 mcg/min) = beta-1 stimulation
-When administered in large dosages (10-10 mcg/min) = alpha-1 stimulation
Beta-1 stimulation = + inotropic (increased contractility) and + chronotropic (increased HR) effect thus
resulting in increased SBP, increased HR, and increased myocardial 02 consumption.
Beta-2 stimulation = relaxation of bronchial, skeletal, and smooth muscle vasculature which results in
decreased SVR, decreased afterload, and decreased DBP
*Net effect: INCREASED pulse pressure with minimal changes in MAP with little likelihood of
baroreceptor mediated reflex bradycardia
*The increased HR, increased myocardial contraction, and increased venous return = increased CO
Alpha-1 stimulation = intense vasoconstriction with increased SVR, increased afterload, and increased
BP (in high doses).
DOSAGE: 1-4 mcg/min (small to moderate) or 0.1 mcg/kg/min
USES: treatment of anaphylaxis, hypotension, adjunct therapy in CPR, acute asthma exacerbation
CONTRAINDICATIONS: hypertension, arrhythmias, and cerebrovascular disease
ADVERSE EFFECTS: hypertension, arrhythmias, angina, tissue necrosis with infiltration, and cerebral hemorrhage, tachycardia
METABOLISM: Epinephrine as well as Norepinephrine are metabolized by catechol-O-methytransferase (COMT) and monoamine oxidase (MAO). The final product of these pathways is vanillylmandelic acid (VMA) and is excreted in the urine.
VASOPRESSIN (arginine vasopressin (AVP); antidiuretic hormone (ADH))
Vasopressin is a hormone formed by the hypothalmus and released from the posterior pituitary gland. It plays a role in maintenance of blood pressure through its ability to regulate water reabsorption by the kidney. It is also a potent vasoconstrictor.


When administered exogenously, vasopressin interacts with V-1 receptors and V-2 receptors.
V-1 receptors are located in vascular smooth muscle, bladder, hepatocytes, and renal cells. (treatment
for shock (septic or hypovolemic). Vasopressin helps maintain BP during shock via the
vasoconstriction effects of V-1 stimulation.
V-2 receptors are located predominantly in the renal collection duct system. (vasopressin/ DDAVP is
the treatment of choice for pituitary diabetes insipidus and hemorrhage). Additional maintenance of
vascular tone is due to the increasing water reabsorption effects of V-2 stimulation.
Vasopressin has also shown to produce vasodilation in the renal, pulmonary, and mesenteric systems via endothelial nitric oxide release.
MECHANISMS OF ACTION: There are several mechanisms regulating the release of AVP. Hypovolemia, as occurs during hemorrhage, results in a decrease in atrial pressure. Specialized stretch receptors within the atrial walls and large veins (cardiopulmonary baroreceptors) entering the atria decrease their firing rate when there is a fall in atrial pressure. Afferent nerve fibers from these receptors synapse within the nucleus tractus solitarius of the medulla, which sends fibers to the hypothalamus, a region of the brain that controls AVP release by the pituitary. Atrial receptor firing normally inhibits the release of AVP by the posterior pituitary. With hypovolemia or decreased central venous pressure (CVP), the decreased firing of atrial stretch receptors leads to an increase in AVP release. Hypothalamic osmoreceptors sense extracellular osmolarity and stimulate AVP release when osmolarity rises, as occurs with dehydration. Finally, angiotensin II receptors located in a region of the hypothalamus regulate AVP release – an increase in angiotensin II simulates AVP release.
AVP has two principal sites of action: the kidney and blood vessels. The most important physiological action of AVP is to increase water reabsorption in the kidneys by increasing water permeability in the collecting duct, thereby permitting the formation of a more concentrated urine. This is the antidiuretic effect of AVP and it acts through vasopressin type 2 (V2) receptors coupled to adenylyl cyclase. AVP also constricts arterial blood vessels by binding to V1receptors, which are coupled to the Gq-protein and the phospholipase C/IP3 signal transduction pathway. Normal physiological concentrations of AVP are below its vasoactive range; however, in hypovolemic shock when AVP release is very high, AVP does contribute to the compensatory increase in systemic vascular resistance.
V-1 receptor (constriction)
1. vasopressin binds with V-1 receptor
2. Gq protein is activated
3. GTP replaced GDP on alpha-subunit
4. Phospholipase C is activated
5. IP3 and DAG are released
DAG activates Protein Kinase C
IP3 releases free calcium from stored calcium
6. Calcium binds with Calmodulin
7. This complex activates Myosin Kinase which phospholates the myosin head
8. Actin and Myosin connect = contraction = constriction
Vasodilation
1. Nitric oxide (NO) activates guanylyl cyclase
2. Guanylyl cyclase converts GTP to cGMP
3. cGMP stimulates Protein Kinase G
4. Actin and myosin interaction is inhibited
5. cGMP also inhibits calcium entry into smooth muscle = muscle relaxation.
USES: treating excessive water loss caused by diabetes insipidus; bleeding caused by esophageal varices, and shock. Vasopressin has also shown to produce vasodilation in the renal, pulmonary, and mesenteric systems via endothelial nitric oxide release.
DOSAGE: 0.0012-0.2 units/kg/min
ADVERSE EFFECTS: coronary vasoconstriction (high doses) leading to angina, ischemia, or injury (administer to patients cautiously with CAD); hypertension, headache, nausea, bronchoconstriction, abdominal cramps, and ventricular dysrhythmias.
CONTRAINDICATIONS: Severe liver and renal function, advanced artherosclerosis, and poor cardiac function due to increased SVR from V-1 stimulation.
NEOSYNEPHRINE (PHENYLEPHRINE)
vasopressor
Phenylephrine is a sympathomimetic amine which has primary effect on alpha adrenergic receptors. It is used to produce vasoconstriction as an adjunct to correct hemodynamic imbalances.
ACTION: Neosynephrine binds with alpha-1 receptor causing a change to occur in the G-protein on the inside of the cell membrane that is coupled with the alpha-1 receptor. This change consists of GDP in the G-protein alpha subunit converting to GTP and simultaneously the alpha-GTP subunit of the G-protein becoming detached. The alpha-GTP complex then migrates to and binds with Phospholipase C. Intrinsic GTPase activity converts GTP back to GDP and the alpha subunit returns to the inactive state. Activated phospholipase C catalyzes the production of DAG and IP3. Increased levels of DAG and IP3 ultimately lead to increased cellular calcium ions and regulation of protein kinase C, which regulates the final physiologic action on vascular smooth muscle which is contraction.
1. Neo binds with alpha-1 receptor
2. This causes a change in G-protein
3. GDP converts to GTP on the alpha-subunit
4. Phospholipase C is activated
5. DAG and IP3 are activated
6. Increased levels of DAG and IP3 cause calcium to move into the cell = contraction
*The outcomes are arterial constriction and increased SVR/afterload, increased venous constriction, increased venous return, increased LV preload, increased ventricular stretch, increased strength of contraction, increased SV and CO and BP.
USES: Hypotension resulting from cardiogenic shock, circulatory shock (including septic), hemorrhagic shock, and hypotension that occasionally occurs from anesthesia.
DOSAGE: 10-300 mcg/min
ADVERSE EFFECTS: headache, reflex bradycardia (results from increased vagal activity as a reflex to increased arterial blood pressure), HTN, decreased tissue perfusion. Use in caution with patients with CAD, acute MI, CHF, and other heart conditions.
DOPAMINE
inotrope, vasopressor

Dopamine is a catacholamine that when administered exogenously has dose dependent dopaminergic, beta, and alpha effects. It is the immediate precursor of norepinephrine. Clinically, dopamine is unique due to its ability to simultaneously increase myocardial contractility, cardiac output, renal blood flow, GFR, and urine output. Doses for dopamine are dependent on desired outcomes.
USES: adjunct to standard measures to improve: Blood pressure, cardiac output, urine output in treatment of shock unresponsive to fluid replacement.
DOSES:
Small doses (0.5-3 mcg/kg/min) stimulate dopaminergic receptors, producing renal vasodilation
Moderate doses (3-10 mcg/kg/min) produce + inotropic and + chronotropic effects through
stimulation of Beta-1 receptors
High doses (>10 mcg/kg/min) stimulate alpha-1 receptors leading to vasoconstriction
CONTRAINDICATIONS: tachyarrhythmias, pheochromocytoma (a rare tumor of neuroendocrine origin, found in children and adults and is a cause of essential hypertension), and V-fib.
USE CAUTIOUSLY IN... hypovolemia, myocardial infarction, occlusive vascular diseases, pregnancy, lactation, and children
ADVERSE REACTIONS: tachycardia, digital gangrene, extravasation necrosis, and ventricular arrhythmias
METABOLISM: Dopamine is metabolized by catechol-O-methytransferase (COMT) and monoamine oxidase (MAO) into homvanillic acid and excreted in the urine
ALPHA RECEPTOR
1. Dopamine binds with alpha-1 receptor
2. G protein is activated
3. GTP replaces GDP
4. Phospholipase C is activated
5. IP3 and DAG are activated. DAG activates the protein kinases
IP3 releases stored calcium leading to an increased concentration of free calcium
6. Free calcium binds with calmodulin
7. This complex activates myosin light chain kinase
8. The myosin light chain kinase phospholates the myosin chains on the myosin head in the presence of
ATP
9. This leads to a cross-bridge formation between the myosin heads and actin filaments causing smooth muscle contraction = constriction
BETA RECEPTOR
1. Dopamine binds with beta-1 receptor
2. Gs protein is activated
3. Gs protein activates adenylyl cyclase to form cAMP from ATP
4. cAMP activates protein kinase-A
5. Protein kinase-A phospholates calcium channels increasing calcium entry into the cells
6. Increasing calcium during action potentials leads to increased calcium release by the sarcoplasmic reticulum in the heart (increasing contractibility, aka inotropy). Gs protein also increases HR by opening ion-channels responsible for pacemaker currents in SA node.
7. Protein Kinase-A phospholates sites on the sarcoplasmic reticulum which increases the release of calcium.
8. Calcium binds with triponin-C which enhances inotropy
9. Protein kinase-A phospholates myosin light chains. Myosin binds with actin. Myosin head flips forward causing contraction.
10. Juxtamedullary cells: angiotensin II causes adrenal cortex to release aldosterone = increased sodium
and water reabsorption

D-1 RECEPTOR
Stimulation of dopamine-1 receptors in the renal tubules stimulates natriuresis. Dopamine-1 receptors stimulate adenylate cyclase and phospholipase and the formation of cyclic adenosine monophosphate. In the renal vasculature, dopamine-1 receptors mediate vasodilation and increase renal blood flow and glomerular filtration rate. In renal tubules, stimulation of dopamine-1 receptors inhibits sodium reabsorption and promotes natriuresis.
NITROGLYCERIN
Classification: antianginal, nitrate
USES: Acute (translingual and SL) and long-term prophylatic (oral, buccal, transdermal) management of angina pectoris.
PO: adjunct treatment of CHF.
IV: adjunct treatment of acute MI. Production of controlled hypotension during surgical procedures. Treatment of CHF associated with acute MI. Management of chronic CHF
ACTION: increases coronary blood flow by dilating coronary arteries and improving collateral flow to ischemic regions. Produces vasodilation. Decreases left ventricular-end-diastolic pressure and left ventricular-end-diastolic volume (preload). Reduces myocardial consumption. Relieves or prevents anginal attacks. Increases cardiac output. Reduction of blood pressure.
DOSAGE: 5-200 mcg/min IV
CONTRAINDICATIONS: hypersensitivity, severe anemia, pericardial tamponade, constrictive pericarditis, hypotension, hypovolemia, aortic stenosis
ADVERSE REACTIONS: hypotension, headache, flushing, reflex tachycardia, nausea/vomitting.
Excessive doses may cause increased methemoglobin concentrations (methemoglobinemia) tx: methylene blue
The result of methemoglobinemia is that oxygen delivery to tissues is impaired and the oxygen hemoglobin dissociation curve shifts to the left.
METABOLISM: undergoes rapid and almost complete metabolism by the liver; and also metabolized by enzymes in the blood stream
------------------------------------------------------------------------------------------------------------------------------
Now I am going to talk about common disorders/disease processes that is seen in the ICU.....
SHOCK SYNDROMES
Circulatory shock is characterized by inadequate tissue blood flow and O2 delivery to cells, resulting in generalized deterioration of organ function. The usual cause of inadequate tissue perfusion is inadequate cardiac output due to decreased venous return or myocardial depression. Decreased cardiac output associated with shock decreases tissue oxygen delivery, which in turn decreases the level of metabolism that can be maintained by different cells of the body. Skeletal muscle weakness is prominent, reflecting inadequate delivery of oxygen to these tissues. Metabolism is depressed, and the amount of heat liberated is decreased. As a result, body temperature tends to decrease.
In the early stages of shock, consciousness is usually maintained although mental clarity may be impaired. Consciousness is likely to be lost as shock progresses. Low cardiac output greatly decreases urine output or can even cause anuria because glomerular pressure decreases below the critical value required for filtration of fluid into Bowman's capsule. Furthermore, the kidneys have such a high rate of metabolism and require such a large amount of nutrients that decreased renal blood flow may cause acute tubular necrosis. An important feature of persistent shock is eventual progressive deterioration of the heart. In addition to myocardial depression caused by the decreased coronary artery blood flow, the myocardium can also be depressed by lactic acid, bacterial endotoxins, and myocardial depressant factor released from an ischemic pancreas.
HEMORRHAGIC SHOCK
Hemorrhagic is the most common cause of shock due to decreased venous return. Any decrease in systemic blood pressure initiates powerful baroreceptor-mediated increases in sympathetic nervous system activity, manifesting as arterial constriction, venoconstriction, and direct myocardial stimulation. Venoconstriction is particularly important for sustaining venous return to the heart and, thus, maintaining cardiac output. Arterial constriction is responsible for initially maintaining systemic blood pressure despite decreases in cardiac output. This maintenance of systemic blood pressure sustains cerebral and coronary artery blood flow because significant vasoconstriction does not occur in these organs. In other organs, such as the kidneys, intense sympathetic nervous system-mediated vasoconstriction may decrease blood flow dramatically. Complications associated with hypovolemic shock depend on the length of time and severity of the hypotensive crisis. Complications may range from renal damage to cerebral anoxia and death.
Decreased BP, Decreased CO, Decreased CVP, Decreased Wedge, and Increased PVR
Management focuses on restoring circulating volume and resolving the cause of volume loss. Composition of volume replacement therapy depends on what was lost. Crystalloid solutions are used primarily as first-line therapy. Isotonic solutions such as LR or 0.9% NS are preferred over hypotonic solutions (D5). Blood products and other colloid solutions (albumin and synthetic volume expanders) may be used to assist in the resuscitation process, especially if blood loss is the primary cause. The use of RBCs is of utmost importance if hypotension is due to hemorrhage, and they may be useful in other hypovolemic states. The use of colloids in the early phase of fluid replacement is somewhat controversial. During shock, a state of increased capillary membrane permeability causes a shift of intravascular volume into the extravascular space. However, in some cases, colloids may enter the extravascular space, further shifting fluids from the intravascular space to the extravascular space and thereby worsening hypovolemia. The existing research data do not show a reduction in mortality or other complications of resuscitation with the use of colloids.
CARDIOGENIC SHOCK

Cardiogenic shock is actually extreme CHF and therefore results from loss of critical contractile function of the heart. Usually, cardiogenic shock is diagnosed by the presence of systemic and pulmonary hemodynamic alterations, which result from inadequate cardiac output and tissue perfusion. Typically, this occurs when greater than 40% of ventricular mass is damaged. The most common cause of cardiogenic shock is an extensive left ventricular myocardial infarction. In-hospital mortality rates have declined as a result of early revascularization procedures, defined as the capability to preform cardiac catheterization, percutaneous coronary intervention, and open heart surgery. Although cardiogenic shock may develop within a few hours after the onset of myocardial infarction symptoms, it often occurs after hospitalization. Other causes include papillary muscle rupture, ventricular septal rupture, cardiomyopathy, acute myocarditis, valvular disease, and dysrhythmias.
Risk factors: increased age, left ventricular ejection fraction < 35% on hospital admission, large myocardial infarction, history of diabetes mellitus, and previous myocardial infarction.
Patho: Cardiogenic shock is caused by loss of ventricular contraction force, which results in decreased stroke volume and decreased cardiac output. Neuroendocrine compensatory mechanisms are activated to increase preload through retention of sodium and water. Vasoconstriction also increases afterload (SVR). Ventricular filling pressures increase because of the increased preload, but lack of contractility prevents complete ejection. The ventricle becomes distended, further impairing effective contraction, and cardiac output continues to decrease. Compensatory mechanisms continue the vicious cycle of elevated ventricular filling pressures and SVR in combination with an inability of the heart to eject an adequate volume of blood into circulation. Blood pools in the pulmonary circulation, resulting in pulmonary congestion. Pulmonary capillaries are under increased pressure and leak fluid into the interstitium and alveoli, preventing the diffusion of oxygen from alveoli into the pulmonary capillaries and reducing oxygen tension in the blood. The body's cells become ischemic because of the decreased cardiac output , adding to an already tenuous state of myocardial functioning by further stimulating compensatory mechanisms to increase perfusion into the cells. Increased sympathetic stimulation increases the heart rate even more, further escalating myocardial oxygen demands and compounding the crisis. Associated hypotension prevents adequate oxygenation of myocardial tissue exacerbating the anaerobic metabolism of the myocardial tissue and further decreasing the contractile state of the heart. These stressors placed on the failing heart may result in extension of a myocardial infarction.
Clinical manifestations:
SBP < 90 mm Hg, MAP < 70 mm Hg, Cardiac index <2.2L/min/m2, PA wedge pressure >18mm Hg
Thready, rapid pulse, narrow pulse pressure, distended neck veins, arrhythmias, chest pain, cool moist skin, oliguria, decreased mentation, dyspnea, increased RR, inspiratory crackles, possible wheezing, ABGs showing a decrease in PaO2, respiratory alkalosis, enlarged heart, and pulmonary congestion.
Management is aimed at increasing myocardial oxygenation, maximizing CO, and decreasing LV workload. First goal is to correct the reversible problems, protect ischemic myocardium, and improve tissue perfusion. Reversing the hypoxemia and acidosis can improve the response to other therapies. Fluids should be managed to provide adequate filling pressure without overdistention of the ventricle. Left ventricular filling pressures are often elevated; therefore diuresis or nitrate infusion may be indicated to achieve optimal preload. Electrolytes may need to be replaced to provide optimal conditions for the damaged myocardial muscle. Using narcotic analgesics and sedatives to minimize the sympathetic nervous system response can increase venous capacitance and decrease resistance to ejection. Narcotics also relieve ischemic pain. Increasing the oxygen concentration of inspired air is an important step, but may require initiation of mechanical ventilation.
Correcting dysrhythmias with antidysrhythmic agents, cardioversion, or pacing can help restore a stable heart rhythm and enhance cardiac output. In general a preload of 14-18 should be maintained. Pulmonary artery catheters may be placed for hemodynamic monitoring. Fluids or diuretics may be used depending on the left ventricular filling pressures. Norepinephrine and epinephrine may enhance cardiac output by increasing contractility, HR, or SVR but increase cardiac work. Also, stimulation of B2 with epi may produce dilation in peripheral vascular beds that robs vital organs of blood, therefore use this agent with caution. Agents with positive inotropic effects that have little activity on vasculature tone, such as low dose dopamine, dobutamine, amrinone, and milrinone, are used more frequently. It is also recommended that vasodilators, such as sodium nitroprusside, nitroglycerin, or angiotensin-converting enzyme inhibitors, be administered to reduce SVR and left ventricular end-diastolic pressure in an effort to increase cardiac output and improve left ventricular function. Mechanical support for the failing ventricle includes intra-aortic balloon pump and LV assist device.

DISTRIBUTIVE SHOCK STATES
Distributive shock can be caused by anaphylaxis (anaphylactic shock), loss of sympathetic tone (neurogenic shock), or sepsis (septic shock). The underlying mechanism is decreased venous return as a result of displacement of blood volume away from the heart due to enlargement of the vascular compartment and loss of blood vessel tone. Loss of blood vessel tone occurs as a consequence of a loss of sympathetic innervation to blood vessels (neurogenic shock) or because of the presence of vasodilating substances in the blood (anaphylactic and septic shock). The most common is septic shock.
ANAPHYLAXIS SHOCK

results from an allergic reaction to a specific allergen that evokes a life-threating hypersensitivity response. It is reported that 59% are due to insect venoms, 18% to drugs, and 10% to food. If left untreated, vascular collapse occurs, resulting in greatly decreased tissue perfusion.
Anaphylaxis may either by immunoglobulin E (IgE) or non-IgE mediated. Non-IgE responses occur without the presence of IgE antibodies and are called anaphylactoid reactions. It is thought that direct activation of mediators causes this response. Anaphylactoid reactions are commonly associated with nonsteriodal anti-inflammatory drugs (NSAIDS), including aspirin. If there has been an anaphylactoid reactions to one agent, restrictions should include all NSAIDS because any of them could elicit a second reaction.
IgE-mediated anaphylaxis occurs as a result of the immune response to a specific antigen. The first time the immune system is exposed to the antigen, a very specific IgE antibody is formed and circulates in the blood. When a second exposure to this antigen occurs, the antigen binds to this circulating IgE, which then activates mast cells and basophils, triggering release of histamine, prostaglandins, leukotrienes, and other biochemical mediators that initiate anaphylaxis.
PATHO: The antibody-antigen reaction causes antibody-specific mast cells and basophils to secrete substances such as histamine, leukotrienes, eosinophil chemotactic substance, heparin, prostaglandins neutrophil, chemotactic substance, and platelet-activating factor 2. These substances, particularly histamine, prostaglandins, and leukotrienes, cause systemic vasodilation, increased capillary permeability, bronchoconstriction, coronary vasoconstriction, and urticaria (hives). Platelet-activating factor 2 is thought to be crucial is development of anaphylactic shock and is implicated in the development of hypotension and cardiovascular dysfunction, but its mechanism of action is not clearly understood. Some of the other substances precipitate a continued downward spiral causing myocardial depression, inflammation, excessive mucus secretion, and peripheral vasodilation. The diffuse arterial vasodilation creates a maldistribution of blood volume to tissues, and venous dilation decreases preload, decreasing cardiac output. Increased capillary permeability leads to loss of vascular volume, further decreasing cardiac output and subsequently impairing tissue perfusion. Initial symptoms include itching, urticaria, and some difficulty breathing due to bronchoconstriction. Death due to circulatory collapse or extreme bronchoconstriction may occur within minutes or hours.
MANAGEMENT: Early recognition and treatment is essential. Therapeutic goals include removal of the offending antigen, reversal of effects of the biochemical mediators, and restoration of adequate tissue perfusion. Treatment depends on symptoms. If the symptoms are mild, immediate therapy includes 02 and subcutaneous or IV administration of an antihistamine, such as diphenhydramine, to block the effects of histamine, and possible an epinephrine injection to reverse the vasodilation and bronchocontstriction. For adults, rapid infusion (over 1-3 minutes) of NS IV is given for severe hypotension or if the patient does not respond promptly to epinephrine. Other pharmacotherapy includes corticosteriods, brochodilators, and if necessary, vasoconstrictors and positive inotropic agents. Always monitor ABCs, level of anxiety, and institute comfort measures relating to dermatological manifestations. If the agent is unknown, evaluation for allergies and future risk for anaphylaxis should be completed.
NEUROGENIC SHOCK
Neurogenic shock results from loss or disruption of sympathetic tone, which causes peripheral vasodilation and subsequent decreased tissue perfusion. The disturbance of sympathetic tone may be caused by any event that disrupts the sympathetic nervous system. The most common cause of neurogenic shock is a spinal chord injury above the level T6. Other causes include spinal analgesia, emotional stress, pain, drugs, or other central nervous system problems.
Spinal shock is a condition that occurs immediately or within several hours of the spinal cord injury and is caused by a sudden cessation of impulses from the higher brain centers. Characteristics include loss of motor, sensory, reflex, and autonomic function below the level of injury, with resultant flaccid paralysis. Loss of bowel/bladder function occur. In addition, the body's ability to control temperature (poikilothermia) is lost, and the
 patient's temperature tends to equilibrate with that of external environment.
patient's temperature tends to equilibrate with that of external environment.Neurogenic shock is a condition seen in patients with severe cervical and upper thoracic injuries. It is caused by the loss of sympathetic input to the systemic vasculature of the heart and subsequent decreased PVR. Signs and symptoms include hypotension, bradycardia, and loss of the ability to sweat below the level of injury. The same clinical findings pertaining to spinal shock occur in neurogenic shock. Orthostatic hypotension may also occur in a patient with a spinal cord injury because the patient is unable to compensate for changes in position. The vasoconstriction message from the medulla cannot reach the blood vessels because of the injury
.

PATHO: Blood is supplied to the spinal cord by the anterior and posterior spinal arteries. Extending off the cord are the spinal nerve roots. The spinal cord is enclosed in the vertebral canal, which consists of 33 vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal. These are held in place by muscles, ligaments, and other supporting structures. Injury to the spinal cord that occurs at impact is referred as the PRIMARY INJURY. The vertebrae may be fractured, dislocated, or compressed. The cervical area is the most frequently involved. Equally destructive is the injury or damage to the spinal cord that continues for hours after the trauma. SECONDARY INJURIES result in additional axonal damage and further neurological deficit. Mechanisms of secondary injuries include:
1. Immune cells, which normally do not enter the spinal cord, engulf the area after a spinal cord injury. These cells respond as they normally would to inflammation in other parts of the body. However, some of the immune cells release regulatory chemicals, some of which are harmful to the spinal cord. Free radicals are produced which damage the cell membrane and disrupt the sodium-potassium pump
2. Hypoperfusion of the spinal cord from microscopic hemorrhage and edema leads to ischemia. Ischemic areas develop at the injury site as well as on or two segments above and below the level of injury.
3. The release of catecholamines and vasoactive substances (norepi, serotonin, dopamine, and histamine) contributes to the decreased circulation and cellular perfusion of the spinal cord.
4. The release of excess neurotransmitters results in overexcitation of the nerve cells. Excitotoxicity allows high levels of calcium into the cells, causing further oxidative damage and damage to the mitochondria. Excitotoxicity is thought to damage oligodendrocytes (the cells that produce myelin), leading to demyelinated axons that are unable to conduct impulses.
MANAGEMENT:
First always access ABGs. Provide mechanical ventilation if needed. Hypotension is generally secondary to volume loss from hemorrhage. Fluid resuscitation is accomplished by the use of IV fluids, crystalloids, or blood. Early administration of blood enhances oxygenation and may minimize the the secondary ischemic injury to the spinal cord. It is also important to move the patient off the backboard as soon as possible to minimize the development of ulcers. The administration of high-dose methylprednisolone remains controversial. It reduces swelling and helps minimize secondary injury by reversing the intracellular accumulation of calcium, reducing the risk of cord degeneration and ischemia. However, steroid use has been associated with severe pneumonia and sepsis.
Hypoventilation from inadequate innervation of respiratory muscles is a common problem after spinal cord injury. Assessment of tidal volume, vital capacity, and breath sounds are frequent. Paralytic ileus and gastric dilation may increase pressure on the diaphragm and cause further respiratory compromise. NGT placement may be necessary.
Hypotension and bradycardia may be due to neurogenic shock or hemorrhagic shock. Causes of hemorrhagic shock= intrathoracic, intra-abdominal, or retroperitoneal injury, or pelvic lone bone fractures.
Insertion to an indwelling foley catheter is also necessary to prevent bladder distention secondary from an atonic bladder.
SEPTIC SHOCK

Septic shock is a complex and generalized process that involves all organ systems. Septic shock is initiated by an infection. Infections may be due to invading gram-negative or gram-postive bacteria, fungi, and viruses. In many patients, multiple causative organisms are present. Bacteria may be introduced through the pulmonary system, urinary tract, or gastrointestinal system; through wounds; or through invasive devices. These organisms may directly stimulate the inflammatory response and other aspects of the immune system that activate cytokines, complement, and coagulation systems.
PATHO: Septic shock is the culmination of the complex interactions among invading microorganisms and immune, inflammatory, and coagulation systems, which results in a proinflammatory and procoagulation state. In response to the presence of microorganisms, macrophages and helper (CD4) type 1 helper T (Th1) cells secrete proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-1beta. In addition to activating neutrophils that kills microorganisms, these proinflammatory cytokines also injure endothelial cells. Once injured, endothelial cells release mediators that cause endothelial cells to lose their tight junction between each other, resulting in increased permeability. Protein-rich fluid moves from the vascular space into the interstitial spaces of tissues, including the lungs. The release of anti-inflammatory cytokines also occurs. Th2 cells secrete the anti-inflammatory cytokines interleukin-4 and interleukin-10, which balance the proinflammatory response. But in some patients, these proinflammatory cytokines fail to shut down or control the proinflammatory cytokines, and the "out of control"proinflammatory response activates the coagulation cascade. Another important aspect of sepsis is the imbalance between procoagulant and anticoagulant factors. Procoagulant factors increase and anticoagulant factors decrease. Endotoxins stimulate endothelial cells to release tissue factor, which then activates factors Va and VIIIa of the coagulation cascade. As a result, thrombin converts fibrinogen to fibrin. Fibrin binds to platelet plugs that have adhered to damaged endothelial cells. This forms a stable fibrin clot. These clots from throughout the microvasculature and cause additional injury and ischemia to distal tissues. Normally, anticoagulant factors (protein C, protein S, antithrombin III, tissue factor pathway inhibitor) modulate coagulation. Thrombin binds with thrombomodulin on a specific receptor on endothelial cells. This binding "activates" protein C. Activated protein C then inactivates factors V and VIII and inhibits the synthesis of plasminogenactivator inhibitor, which then allows plasmin to break down the fibrin-platelet clots. Unfortunately, sepsis lowers the levels of these anticoagulant factors, and the net result is a procoagulant state. Recognition that the proinflammtory and procoagulant responses result in a loss of homeostasis of almost every organ system is key to understanding sepsis.
CARDIOVASCULAR ALTERATIONS: In general, septic shock is associated with three major pathophysiological effects on the cardiovascular system: vasodilation, maldistribution of blood flow, and myocardial depression.
Proinflammatory cytokines stimulate the release of nitric oxide from endothelial cells. Nitric oxide is a potent vasodilator and causes widespread vasodilation. This vasodilation is often resistant to vasopressor agents. Because of this vasodilation, there is decreased venous return to the heart, decreased cardiac output, and decreased systemic vascular resistance (SVR).
Other inflammatory mediators, including tumor necrosis factor-alpha and endothelin, cause vasoconstriction in other vasculature beds. This situation of mixed vasodilation and vasoconstriction produced maldistribution of blood flow, particularly in the microcirculation. Such maldistribution also occurs as inflammatory mediators and endotoxins damage endothelial cells. This initiates the coagulation cascade within the microvasculature. Small clots form, causing hypoxia to cells distal to the obstruction. This is pivotal to the progression from sepsis to septic shock, SIRS, MODS, and death. In septic shock, evidence of depressed myocardial performance occurs in the form of decreased ventricular ejection fraction, dilation of the ventricles, and a flattening of the Frank-Starling curve after fluid resuscitation. This myocardial depression is not caused by myocardial ischemia but rather myocardial depressant factors released as part of the inflammatory cascade and generation of nitric oxide. Lactic acidosis, which decreases myocardial responsiveness to catecholamines, may also be partly responsible. The heart demonstrates impaired contractility and ventricular performance.
Early in septic shock, it is believed that the heart is hyperdynamic, which high cardiac output and low SVR. However, even at this stage the heart is performing less that optimally. Later, as circulating cardiac depressants increase, the heart becomes hypodynamic, with low cardiac output and increased SVR.
PULMONARY ALTERATIONS: Activation of the sympathetic nervous system and release of epinephrine from the adrenal medulla produce bronchodilation. However, this may be overridden by activity of cytokines, and the net result is bronchoconstriction. More important, inflamatory mediators and activated neutrophils cause capillary leak into the pulmonary interstitium, resulting in interstitial edema, areas of poor pulmonary hypertension, and increased respiratory work. As fluid collects in the interstitium, pulmonary compliance is reduced, gas exchange is impaired, and hypoxemia results. These pulmonary alterations may culminate ARDS, which is frequently associated with septic shock. Continued fluid accumulation in the pulmonary interstitium may finally spill over in the alveoli, producing alveolar infiltrates that produce fertile areas for bacterial growth. Mechanical ventilation may provide an avenue of entry for lung infections. Therefore, a secondary pneumonia may develop possibly caused by a different organism than what produced the sepsis.
HEMATOLOGICAL ALTERATIONS: Platelet abnormalities also occur in septic shock because endotoxin indirectly causes platelet aggregation and subsequent release of more vasoactive substances (serotonin and thromboxane A2). Circulating platelet aggregates have been identified in the microvascular of septic patients. These cause obstruction to blood flow and compromised cellular metabolism. Overactivation of the coagulation cascade without counterbalance of adequate fibrinolysis compromises tissue perfusion both regionally and globally. Over time, clotting factors are depleted, and a coagulopathy results, with a potential of progressing to disseminated intravascular coagulation (DIC).
METABOLIC ALTERATIONS: excessive catecholamine release stimulates gluconeogensis and insulin resitance, which both result in hyperglycemia in critically ill patients who do not have diabetes. Cells are progressively unable to use glucose, protein, and fat as energy sources. Hyperglycemia that is resistant to insulin therapy is a frequent finding in early shock. Eventually, all glycogen energy stores are depleted, cells lack ATP, and cellular pumps fail, progressing to tissue and organ death.
In response to lack of effect of insulin, proteins break down, as shown by high blood urea nitrogen and urinary nitrogen excretion. Muscle protein is broken down into amino acids, some of which are used as energy sources for the Krebs cycle or as substrates for gluconeogensis. In later stages of shock, the liver is unable to use the amino acids because of its own metabolic dysfunction. Amino acids then accumulate in the bloodstream. As shock progresses, adipose tissue is broken down to furnish the liver with lipids for energy production. Hepatic triglyceride metabolism produces ketones, which circulate to peripheral cells that can use them in the Krebs cycle for ATP production. However, as liver function decreases, triglycerides are not broken down; they collect in the mitochondria, inhibiting the Krebs cycle and resulting in increased anaerobic metabolism and lactate production. The ability of cells to extract or use oxygen is impaired as a result of mitochondria dysfunction. Oxidants are normally produced as a byproduct of oxidative phosphorylation. However, in critical illness, and accumulation of oxidants occurs that results in oxidative stress. Oxidative stress causes lipid peroxidation, protein oxidation, and mutations in mitochondiral DNA, thus contributing to cell death. The net effect of these metabolic derangements is that cells become energy starved. This energy deficit is implicated in the emergence of multiple organ failure that frequently develops regardless of interventions designed to support the circulatory and organ systems.
PHYSICAL FINDINGS: some of the earliest signs include altered mentation, increased RR as compensation for metabolic acidosis, and either fever or hypothermia. Because of the exaggerated inflammatory response with release of vasoactive mediators, the clinical presentation of the patient is complex. The patient is edematous yet intravascularly depleted, and areas of microthrombi and vasoconstriction obstruct perfusion. As fluid replacement occurs, the leaking capillary beds shift the fluid interstitially, requiring more fluid resuscitation, which may further exacerbate interstitial edema. Because of the inappropriate systemic activation of the coagulation system, clotting factors are depleted, and spontaneous bleeding may occur. Perfusion imbalances cause ischemia in some vascular beds, such as the splanchnic circulation, skin, and extremities, which may lead to necrosis. Cardiac output may be unusually high, but it is insufficient to maintain adequate perfusion due to circulating myocardial depressant factors. Compensatory mechanisms, such as activation of the sympathetic nervous system, continue to increase cardiac output. However, inflammatory mediators prevent necessary vasoconstriction, and the SVR remains inappropriately low, thereby perpetuating the hypoperfusion crisis.
MANAGEMENT: the primary goals of treatment are to maximize oxygen delivery above cellular oxygen consumption requirements and to halt the exaggerated inflammatory response. Adequate volume replacement is important for reversing hypotension. Patients may require several liters or more of fluid because of mediator-induced vasodilation and capillary leak. PA caths and A-lines may be placed for close monitoring. Vasoactive drugs may be administered to support circulation. Always, maintaining adequate ventilation is important.
DISSEMINATED INTRAVASCULAR COAGULATION (DIC)
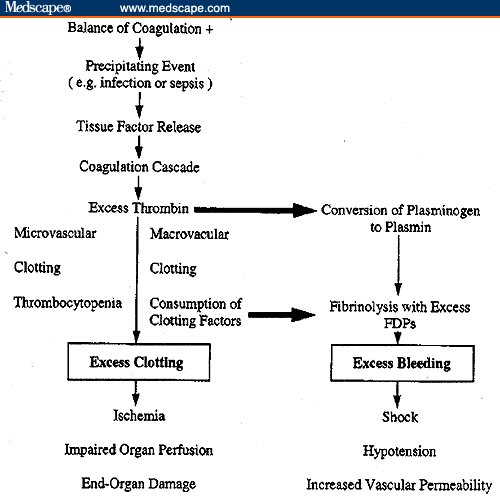
DIC is defined as the inappropriate triggering of the coagulation cascade and a breakdown in the normal feedback mechanisms in the body that allow for the dissolution of clots. Instead of a localized response to tissue damage or vascular injury, there is systemic coagulation activity, resulting in diffuse intravascular fibrin formation and widespread intravascular clotting. Eventually, coagulation factors become depleted as the body attempts to dissolve the newly formed clots. Because of the rapidity of intravascular thrombin formation, clotting factors are used up at rate exceeding replenishment. In essence, there is an imbalance between the natural procoagulant and anticoagulant systems in the body. The result is unregulated thrombin activity, microvasculature thrombi, platelet consumption, and microangiopathic hemolytic anemia.
Activation of coagulation mechanisms also activates the fibrinolytic system. The breakdown of fibrin and fibrinogen results in fibrin degradation products and d-dimer. These products interfere with platelet function and the formation of the fibrin clot. In addition, plasmin can activate the complement and kinin systems, leading to increased vascular permeability, hypotension, and shock. Thus, the patient has simultaneous, self-perpetuating combination of thrombotic and bleeding activity occurring in response to the precipitating event, as well as the potential for hemodynamic instability.
CAUSES: The extrinsic pathway is activated by damage to the endothelial lining of blood vessels. Common causes include surgery, burns, heat stroke, bacterial endotoxin, and malignant tumors. Endotoxins released by gram-negative bacteria resulting in sepsis account for 20%. Shock or low-flow states can result in metabolic acidosis, tissue ischemia, and necrosis, which may also lead to clot formation. In cancer, a common etiology of of DIC, the condition is caused by tumors eroding tissue with subsequent release of thromboplastin or stimulation of factor XII from vascular injury, as well as by the autolysis of tumor cells in rapidly proliferating tumors. In cancers that are able to autolyse (acute leukemia, Burkitt's lymphoma, small cell lung cancer), the cell fragments that result from the lysis are seen as "foreign bodies" and stimulate clotting. Still other cancers release procoagulants that enhance clotting (mucin-producing adenocarcinomas, prostate and renal cancer, promyelocytic leukemias, and brain tumors).
SIGNS/SYMPTOMS: the nurse evaluates the patient for signs and symptoms of inappropriate clotting: cyanosis, gangrene, mental status changes, altered level of consciousness, cerebrovascular accident, pulmonary embolus, bowel ischemia and infarction, and renal insufficiency or failure. Thrombosis may involve both arteries and veins, and clinical examination may damarcation cyanosis (total occlusion of microvessels, most common in digits but may be evident in earlobes). In addition, the nurse evaluates for signs of bleeding: bleeding from the nose, gums, lungs, GI tract, surgical sites, injection sites, and intravascular access sites; hematuria; acral cyanosis; petechial rashes; and purpura fulminans.
MANAGEMENT: the backbone of therapy for DIC is elimination of the causative agent. Antibiotic or antifungal therapy in sepsis, antineoplastic therapy, rehydration, increasing oxygenation, or resolution of low-flow states.
Patients at risk for development for DIC as a result of sepsis may be given activated protein C to slow the development of uncontrolled clotting that may result from the sepsis process.
Heparin therapy may be initiated to minimize further clotting by increasing neutralization of thrombin. However, the risk for increased bleeding is always a major concern. In acute DIC, few clinical studies have shown heparin's effectiveness in slowing coagulation cascade.
Replacement therapy and repletion of clotting factors are the focus of treatment for significant hemorrhage. Fresh frozen plasma contains components of both the coagulation and fibrinolytic systems and can be given to normalize the INR. The recommended dose is 10-20 ml/kg. Platelet transfusions are usually used only for patients with active bleeding or a platelet count < 20,000/mm3. Cryoprecipitate may be used for patients with plasma fibrinogen levels <100 mg/dL. RBC transfusions, although not useful for repleting coagulation factors, may be given to increase hemoglobin and oxygen-carrying capacity.
VENTILATORS
Goals: maintain airway, decrease the work of breathing, support/improve oxygenation, clear secretions.
Types of artificial ventilation:
1. Negative Pressure: most like the body's normal process.
2. Postive Pressure: Blowing air into airway
Positive Pressure Ventilator Modes
1. Control: Original mode, only used in OR/anesthesia. Used for someone who has NO ability
to breath on their own. Delivers preset number of breaths at a preset percentage of oxygen
2. Assist Control: Delivers preset number of breaths at a preset tidal volume and percentage of
oxygen but allows patient to take a breath on their own. Useful for those who are fatigued.
However, if patient starts to breath a lot on their own, respiratory alkalosis can develop.
3. IMV/SIMV: intermittent mandatory ventilation/synchronized intermittent mandatory ventilation.
It preserves and drives muscle strength. IMV: preset rate, preset TV, may breath spontaneously in
between breaths. SIMV: preset rate, preset TV, breaths are synchronized with patient breaths.
4. Pressure Support: overcomes tube resistance. Can be used as a ventilator mode or in conjunction.
Applies pressure in airway. When a patient spontaneously takes a breath, it makes it easier.
5. CPAP: Continuous positive airway pressure. Patient has to do ALL the work, no set rates,
however has preset oxygenation. Can be used in intubated and non-intubated patients
6. PCV: Pressure control ventilation. Applying pressure on patient inspired airway and maintain
pressure for a set time. Can make inhalation longer than expiration. Sometimes used with ARDS
7. PRVC: Delivers a set TV at the lowest possible pressure. Automatically adjusts to compliance
without need for compliance.
8. Volume Control: set tidal volume
9. PEEP: Positive end expiratory pressure. normal: 5-10. Maintains pressure to keep alveoli open at
all times so they don't collapse. Watch blood pressure and cardiac output as they may decrease. Also
can cause barotrauma, rupture
10. Minute Volume: rate x TV
Complications of ventilators
pneumothorax/barotrauma: increased peep or inflation pressures
Infection/sepsis: VAP
Decreased BP/CO: due to peep especially if already hypovolemic.
Airway malfunction: trauma to airway (teeth knocked out, tearing of oral cavity, tracheal-esophageal
fistula, erosion of trachea)
Acid-base imbalance
Arrhythmias: due to hypoxia. You'll see PVC's and V-tach
Atelectasis: collapse
Stress ulcers/GI bleed: Decreased GI motility, blood is shunted away from gut
02 toxicity: especially is FIO2 > 50% x 2-3 days
Psychological stress: anxiety
If your patient appears....
acidotic and hypercapneic: increase TV, IMV, or MV
alkalotic or hypocapneic: decrease TV, IMV, or MV
Decrased pO2: increase PEEP, Fi02, PIP
-----------------------------------------------------------------------------------------------------------------------------------
As stated before, I followed a CRNA in an OB setting. I started out the day following my CRNA learning the most important lesson of the day, first thing... put on your mascara! Then we went and talked to our patient and our patient's families and explained to them what to expect. We then went and set up our operating room. We grabbed our drug kit and set up the OR. We made sure we had everything we needed just in case something happened (ambu bags, intubation kits, drugs, etc). We would then go get our patient and take her (the soon to be mom) back and start her spinal or epidural, place a foley catheter, lay her down, then we will go get dad.
A spinal anesthetic is placed in the low back (lumbar region). After a sterile prep and draping, local anesthetic is placed in the skin to numb the area where the Spinal needle will be placed. The Spinal needle passes between the vertebrae of the Spinal column through the dural membrane where the cerebroSpinal fluid is located. Once the placement of the needle is accomplished medicines including a local anesthetic and sometimes a narcotic are dispensed via the needle. The needle is then removed. The entire process usually takes anywhere from 5- 20 minutes.
Epidural anesthesia is most commonly placed in the low back (lumbar region). Unlike Spinal this technique may also be accomplished in the mid-back (thoracic region) for surgery in the area of the chest. After a sterile prep and draping, local anesthetic is placed in the skin numb the area where the Epidural need will be placed. The needle for Epidural passes between the vertebrae of the Spinal column to the Epidural space. Once the position is verified, a very small catheter is placed via the needle. The needle is then removed and the catheter remains in the Epidural space. The catheter is then taped to the patients back. Local anesthetics and narcotics given epidurally via this catheter. The procedure usually takes 10 - 25 minutes.
Once the anesthetic has been placed the patient will begin to feel warming of the bottom and legs followed by loss of sensation of the involved area. This is followed by a loss of strength. The time period is anywhere from 5-25 minutes
Postdural puncture headache occurs infrequently with these techniques. The risk seems to be higher with younger age and larger size of the needle. The risks is about 1% with Epidurals and 3% with Spinals. This is believed to be due to a leak of cerebrospinal fluid from the needle hole in the dura. The occurrence of this is greatly reduced by using a smaller needle when possible. If this headache does occur it may be treated initially with hydration and pain medicines. If the headache does not resolve it would be treated with an Epidural blood patch. This if essentially using the patients own blood to block the leak via the Epidural technique.
Another side affect of spinal and epidural anesthesia is hypotension which is why we make sure to give our patient fluids before we begin as well as throughout the procedure. Our patients recieved a total of 2-3 liters.
The day went smoothly and we had no complications. It was a joy to watch and learn from a CRNA and be in the operating room to see things first hand. Here are a few pictures of my day. I have received permission to utilize these pictures in my project, the double-bonus of my day was that I was able to watch my new baby sister being born.... Here are pictures of me shadowing a CRNA and enjoying this special moment with my family. enjoy!
me and my dad

my dad and my new baby sister annabelle


References
Katzung, G. Bertram (2007). Basic and Clinical Pharmacology. Tenth Ed.
New York: McGraw-Hill Company.
Klabunde, E. Richard, Ph.D (2008). Cardiovascular Pharmacology
Concepts. Retrieved October 30, 2010 from
Klabunde, E. Richard, Ph. D (2007). Cardiovascular Physiology Concepts.
Retrieved October 30, 2010 from http://www.cvphysiology.com/.
Morton, G. Patricia, & Fontaine, K. Dorrie (2009). Critical Care Nursing: A
Holistic Approach. Ninth Ed. Philadelphia: Wolters
Kluwer/Lippincott Williams & Wilkins.
Stoelting, K. Robert & Hillier, C. Simon (2006). Pharmacology and
Physiology in Anesthetic Practice. Fourth ed. Philadephia: Lippincott
Williams & Wilkins.